Sleep of Healthy Older Adults
First created | 01/12/1999
Last edited |
Sleep patterns evolve across the normal aging process in complex ways. Chronological age in elderly people does not always match physiological age.
Therefore, changes in sleep patterns may happen earlier, i.e., at a younger age, for some individuals or at an older age for others.
Moreover, epidemiological and other studies suggest that much of the sleep disturbance typically seen in old age is likely the result of medical co-morbidities than age per se.
Nevertheless, four age-related changes have been consistently demonstrated in polysomnographic (PSG) studies of sleep architecture:
- total sleep time,
- sleep efficiency, and
- slow wave sleep all decrease, while
- wake after sleep onset increases with age.
However, a number of PSG sleep characteristics remain uncertain as regard their evolution with age:
- sleep latency has been reported to increase with age in some studies, while several other studies found no significant changes with age).
- percentage of stage 1and
- stage 2 while many others reported an increase with age of these stages).
- Similarly, REM sleep has reported to decrease with age in several studies while many other studies found no such association with age.
Why such discrepancies between the studies?
Several factors may be responsible for the difficulties identifying age trends in sleep architecture of apparently healthy subjects. For example: small sample sizes; inconsistency in controlling factors that may influence sleep, such as mental or physical illness; uncontrolled use of alcohol, drugs or medications; or insufficient screening for sleep disorders.
Epidemiological studies indicate that as much as 50% of the older population complains of significant sleep disturbance.
Numerous age-related sleep changes have been demonstrated both by self report and objective findings:
- increased Time in Bed
- increased Sleep Latency
- increased nighttime wakefulness
- decreased Total Sleep Time
- decreased Sleep Efficiency
- increased Stages 1 and 2
- decreased Stages 3 and 4
- decreased REM(?)
Meta analysis of the "normative" objective sleep literature allows us to:
- determine if these findings are accurate.
- distinguish between age-changes across the adult life-span and age-changes within the older adult portion of the population.
- determine the impact of key variables such as gender and health on objective sleep.
META-ANALYSIS METHODS
PubMed, PsycInfo and SCI searched.
"Sleep" + "normal", "normative" or "healthy".
- 4000+ reports identified and screened.
- Non-clinical participants > five years old.
- Reporting appropriate all-night PSG variables.
- Data presented numerically.
- Peer reviewed and published between 1960 and 2003.
- 585 reports passed screening.
Each screened report was coded for Target variables:
- TST, SL, SE, WASO, REM, S1, S2, S3-4.
- Sample well described, with limitations noted.
- Statistical analytic result for target variables:
- Central tendency, variability and F, t or r value, etc.
- Gender and age of subjects with data summarized accordingly
All the above criteria had to be met for inclusion in the meta-analyses.
Out of 585 reports 65 met all criteria.
Representing a total of 3,577 subjects:
- Eighteen studies assessing the sleep of children (5-18 yrs.), 1,186 subjects.
- Forty-seven studies assessing the sleep of adults (> 19 yrs.), 2,391 subjects.
- Thirty-eight studies assessing the sleep of older adults (60-102 yrs.), 1,142 subjects.
- The median duration of daytime sleep was 0 at all age.
- Long daytime sleep (5% of the subjects): 60 minutes or more in all age groups except in the 35-44 years where the median was 45 minutes
- Effect sizes were measured by calculating the standardized differences between groups using Cohen's d:
- Difference of the group means divided by the pooled standard deviation: (M1-M2)/SD(pooled)
- Effect sizes were analyzed using Comprehensive Meta Analysis (Biostat, Englewood, NJ).
- A Cohen's d ~.2 indicates a small, a ~.5 a medium, and ~.8 a large effect size.
- Positive values indicate an age-related increase and negatives a decrease.
- The Q statistic indicates the likelihood that modifier variables may be impacting the age/sleep relationship: mental illness, physical illness, drugs/alcohol, sleep apnea, other sleep disorders, gender, fixed versus habitual schedule (and school versus non-school day recording).
OVERALL AGE-RELATED TRENDS
| TST | -.76 |
|---|---|
| SE | -.82 |
| S 3-4 | -.56 |
| REM | -.34 |
| REML | -.68 |
| SL | .16 |
| S 1 | .16 |
| S 2 | .34 |
| WASO | .75 |
All variables significant at p<.0001
OVERALL AGE-RELATED EFFECT SIZES
| TST | -.60 |
|---|---|
| SE | -.71 |
| S 3-4 | -.85 |
| REM | -.46 |
| REML | -.15 |
| SL | .27 |
| S 1 | .38 |
| S 2 | .28 |
| WASO | .89 |
All variables significant at p<.0001, except REML, p<.05 · N.B. Each had a highly significant Q statistic.
RESULTS
Clearly sleep patterns change across the full adult life-span.
Do these overall age-related trends continue within the older adult life-span?
What are the age-related changes in sleep patterns, if any, within the older adult (60-102 yrs.) population?
TOTAL SLEEP TIME
(Effect Size = -0.13, CI = -0.35 to 0.08, n.s.)
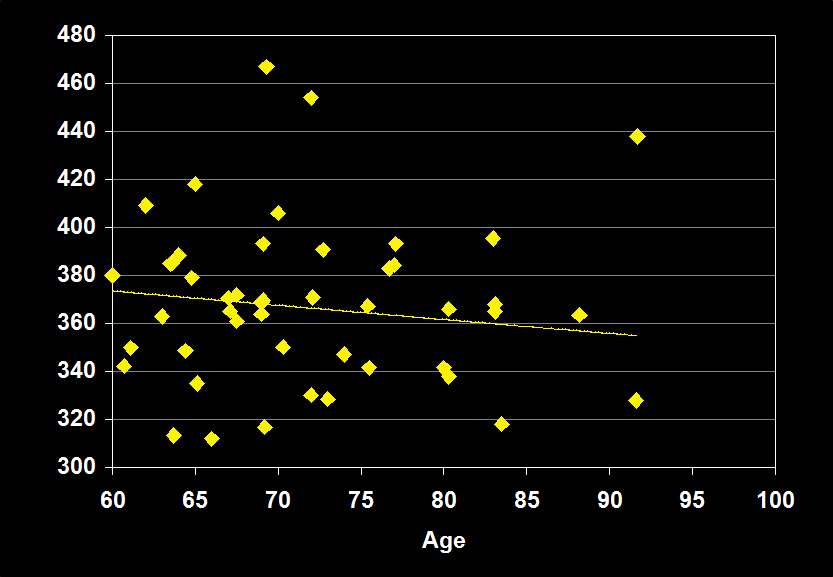
TOTAL SLEEP TIME MODERATORS
- Exclusion of mental and physical illness, alcohol/drugs, sleep apnea and other sleep disorders resulted in increased negative effect sizes.
- Habitual bedtimes increased effect size.
- Effect size was larger for women.
- Effect size was n.s. for subjects over 60: TST does not continue to decline in older adults.
SLEEP EFFICIENCY
(Effect Size = -.41, CI = -.63 to -.19, p < .0001)
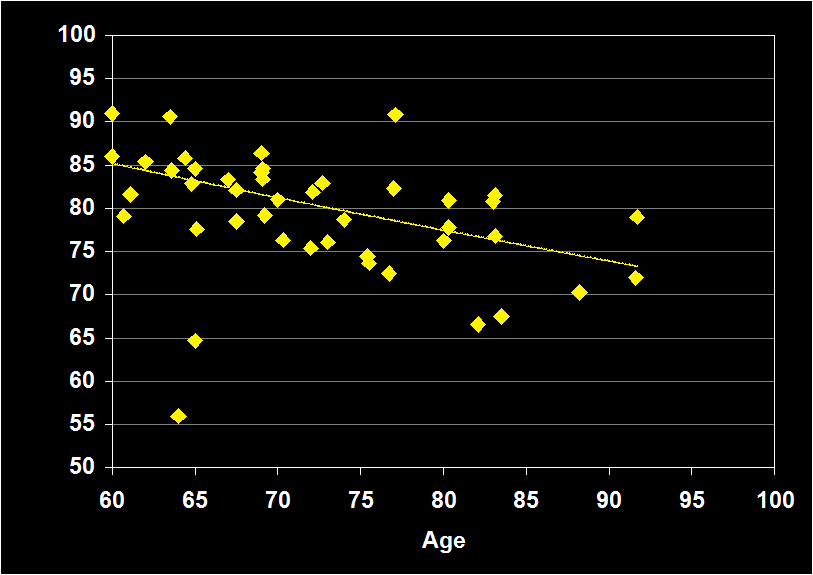
SLEEP EFFICIENCY MODERATORS
- Exclusion of mental and physical illness, sleep apnea and other sleep disorders resulted in increased negative effect sizes.
- Effect size was larger for women.
- Effect size was moderate for subjects over 60 (-.41, CI -.19 to -.63, p<.0001): Sleep Efficiency continues to decline in older adults.
SLEEP LATENCY
(Effect Size = .10, CI = -.20 to .40, n.s)
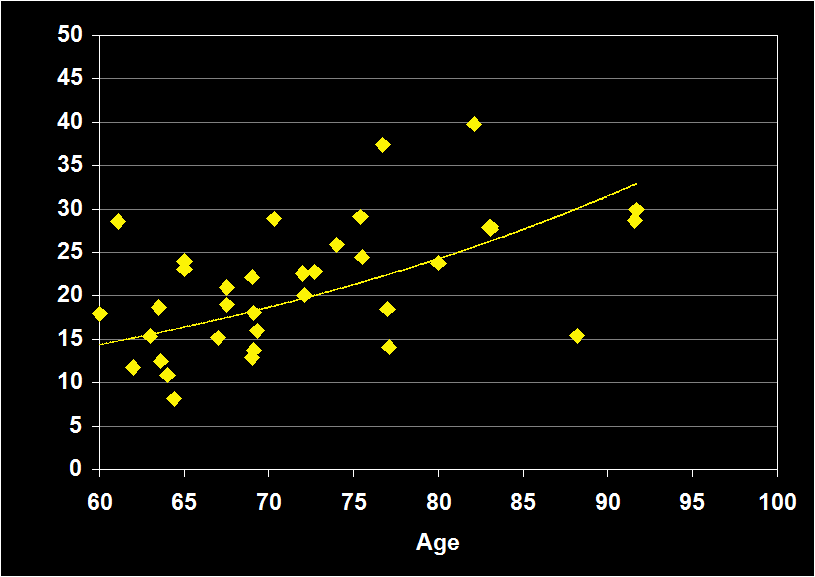
SLEEP EFFICIENCY MODERATORS
- Exclusion of physical illness and other sleep disorders resulted in increased positive effect sizes.
- Studies including sleep apnea had positive effect sizes.
- Effect sizes were n.s. for subjects over 60: SL does not continue to decline in older adults.
PERCENTAGE OF STAGE 1
(Effect Size = .15, CI = -.20 to .50, n.s.)
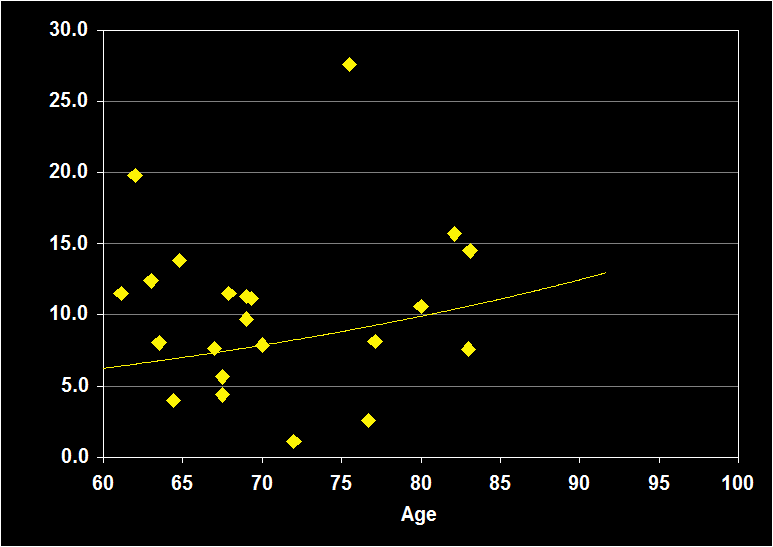
PERCENT STAGE 1 MODERATORS
- Exclusion of mental illness, sleep apnea, other sleep disorders or alcohol/drugs resulted in increased positive effect sizes.
- Physical illness had no impact.
- Larger effect sizes were noted for women.
- Effect sizes were n.s. for subjects over 60: Percent Stage 1 sleep does not continue to decline in older adults.
PERCENTAGE OF STAGE 2
(Effect Size = .02, CI = -.31 to .35, n.s.)
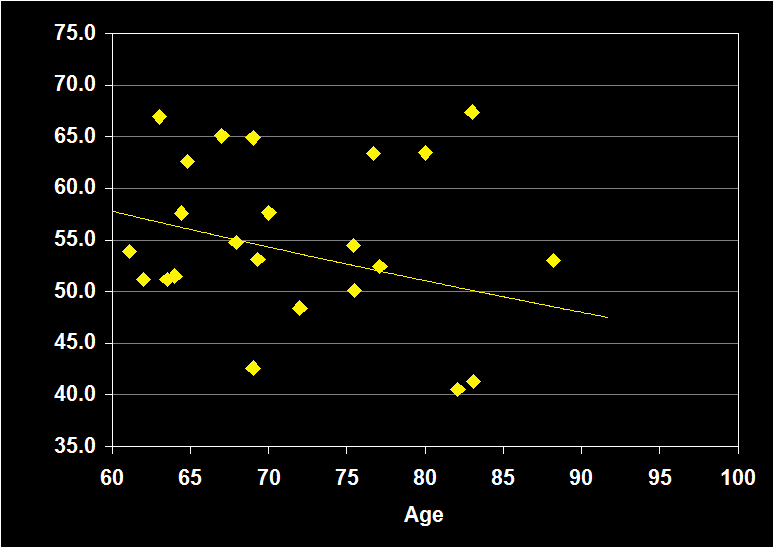
PERCENT STAGE 2 MODERATORS
- Exclusion of mental and physical illness, alcohol/drugs, sleep apnea and other sleep disorders resulted in increased positive effect sizes.
- Habitual bedtimes increased effect size.
- Effect sizes were n.s. for subjects over 60: Percent Stage 2 sleep does not continue to decline in older adults.
PERCENT STAGE 3-4
(Effect Size = -..08, CI = -.41 to .25, n.s.)
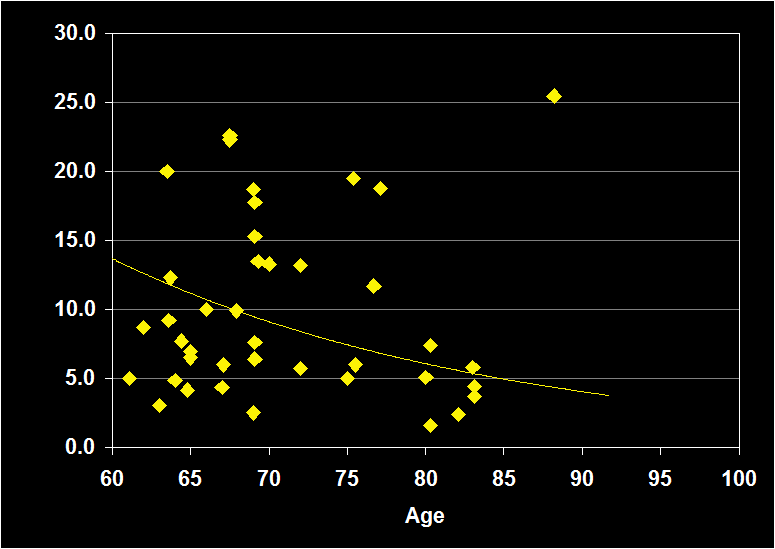
PERCENT STAGE 3-4 MODERATORS
- Exclusion of moderator variables, including gender, did not modify the Percent Stage 3-4 / aging relationship.
- Effect size was n.s. for subjects over 60: Percent Stage 3-4 does not continue to decline in older adults.
PERCENT REM
(Effect Size = -.13, CI = -.35 to .08, n.s.)
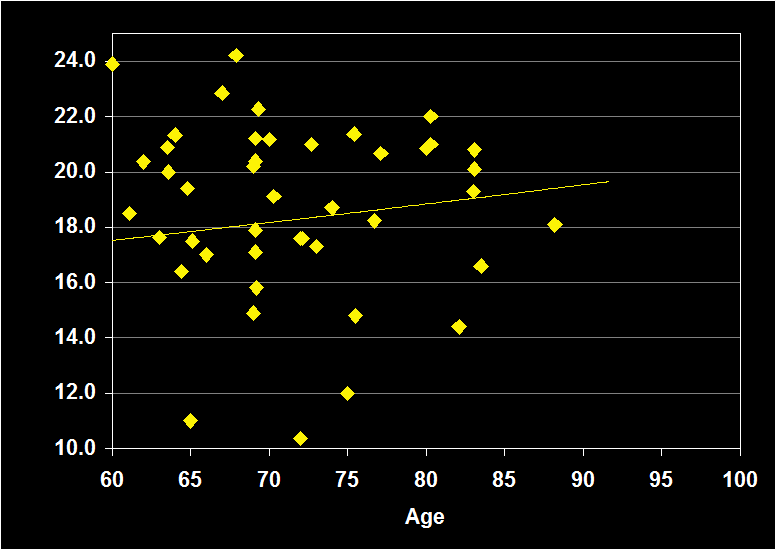
PERCENT REM MODERATORS
- Exclusion of moderator variables, including gender, did not modify the Percent REM / aging relationship.
- Exclusion of other sleep disorders increased the negative effect size.
- Effect size was n.s. for subjects over 60: ·Percent REM does not continue to decline in older adults.
REM LATENCY
(Effect Size = -.04, CI = -.34 to .27, n.s.)
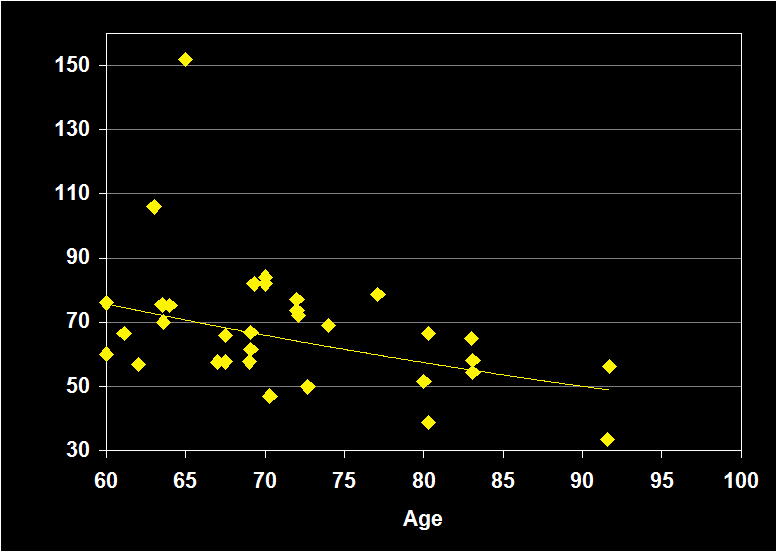
REM LATENCY MODERATORS
- Exclusion of sleep apnea and other sleep disorders resulted in increased negative effect sizes.
- Effect sizes were larger for women in studies using habitual bed times.
- Effect size was n.s. for subjects over 60: REM Latency does not continue to decline in older adults.
WAKE AFTER SLEEP ONSET
(Effect Size = -.08, CI = -.43 to .27, n.s.)
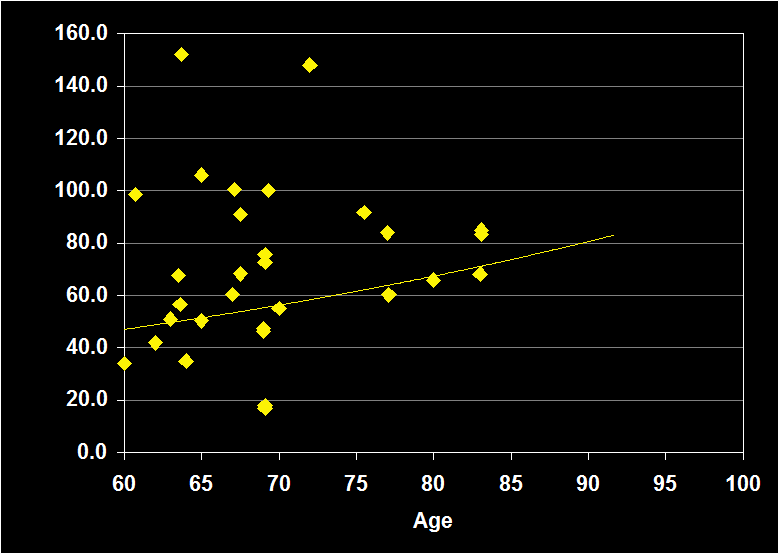
WAKE AFTER SLEEP ONSET MODERATORS
- Exclusion of mental and physical illnesses, sleep apnea and alcohol/drugs resulted in decreased positive effect sizes.
- Exclusion of other sleep disorders resulted in a increased positive effect size.
- Effect sizes were n.s. for subjects over 60: WASO does not continue to decline in older adults.
SUMMARY
- When the full adult life-span of 19 to 102 years was examined:
- TST, SE, S 3-4, REM and REML all declined significantly with advancing age.
- SL, S 1, S 2 and WASO all increased significantly with advancing age.
- Effect sizes tended to increase when various confounds were taken into account.
- However, when only older adults (60-102 yrs.) are examined:
- Only SE changed significantly across the older adult age range, continuing to decline at a rate of ~3% per decade.
- TST, SL, S 1, S 2, S 3-4, REM, REML and WASO did not change significantly with advancing age, despite numerous previous reports of such changes in single studies and review articles.
- Effect sizes tended to be larger for women
- Effect sizes tended to increase, but not to significant levels (except for SE):
- when habitual bedtimes were used.
- when various confounds were taken into account e.g., medical illnesses, sleep disorders, etc...
LIMITATIONS
- For many studies age-related effect sizes could not be calculated for lack of information.
- In general there was a relative dearth of middle-aged subjects, which serves to maximize age-related effect sizes.
- Age-related changes across the life-span are likely subtler than simple young-old group comparisons suggest.
- These findings are based on cross-sectional data: lack of age-related changes in the older adult age range may be a confound of studying "survivors', although analyses took into account medical and mental illnesses as well as sleep disorders.
It remains to be shown if longitudinal data will provide the same answers to these as what is "normative" objective sleep in the older adult age range.
CONCLUSIONS
Accurate "normative" data is necessary to detail what types of sleep patterns can be expected as individuals age and what might be considered pathological deviation from those norms.
This meta analysis clearly illustrates the importance of strict screening for subject health, use of habitual bedtimes, etc. to ensure that data may be considered "normative".
This meta analysis suggests that most age-related objective sleep changes occur in the early and mid years of the human life span and that these changes effectively asymptote in older adults.
In fact, the sleep of healthy older adults remains relatively constant from age 60 to the mid 90s, although older adults can expect their sleep efficiency to decline in a slow but continuous manner.
This lack of significant age-related sleep changes in healthy older adults may well help account for the fact that epidemiological studies report that somewhere between 40 and 60% of older adults complain of significant sleep problems. But it is important to remember that the other half of the population does not complain!
